Knowledge of the protein content in a sample is essential in the laboratory. It allows many other laboratory tests (e.g. SDS-PAGE) to be performed correctly and purification conditions (e.g. chromatographic column loading) to be selected. It is also an important measurement during protein production. It enables the preparation of appropriate dilutions and monitoring of the purification process.

There are several protein determination methods. They use different mechanisms and vary in sensitivity, accuracy, and speed of measurement.
1. UV-VIS Absorbance Measurement at a Wavelength of 280 nm
The basis for the absorbance measurement method at a wavelength of 280 nm is the ability of aromatic amino acids (tryptophan, tyrosine and phenylalanine) to absorb light with a maximum at a wavelength of 280 nm. According to Lambert-Beer’s law, absorbance is proportional to protein concentration (A=еCl). For pure samples, knowing the extinction coefficient, we are able to accurately measure the protein content in the sample.
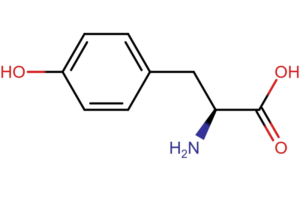
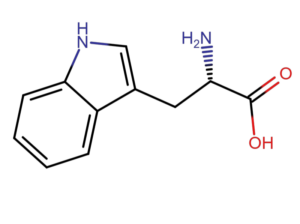
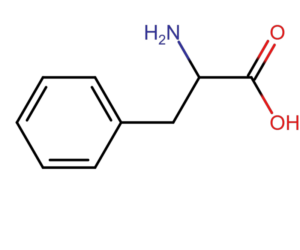
The method of measuring absorbance at a wavelength of 280 nm has a disadvantage. Each protein has a different amino acid composition and a different content of aromatic amino acids. In the case of very complex samples or when amino acids or nucleic acids are present in the matrix, the result is inaccurate.
Microbiuret Method
The microbiuret method is a modification of the traditional biuret method. However, it allows for measurements to be taken on smaller sample volumes. The technique is based on the reaction of peptide bonds with copper ions in a strongly alkaline environment. A minimum of two peptide bonds are required for the reaction to occur. Free amino acids or dipeptides will therefore not give a positive result. Contrary to appearances, the name of the method does not come from burette, but from biuret. Biuret is the simplest compound that gives a positive reaction.
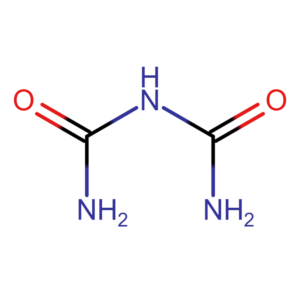
The reaction was described in 1833 by Ferdinand Rosi and independently in 1857 by Gustaw Piotrowski. The result is independent of the amino acid composition of the protein. The reaction produces a coloured copper-protein complex (purple). It exhibits maximum absorption at wavelengths of 310 nm and 505 nm. Measurement at a wavelength of 310 nm may be less sensitive due to the possibility of interference. For this reason, the measurement is often performed at a wavelength of 540 nm.
2. Lowry method
The Lowry method is an improvement on the biuret method. It is based on a two-step chemical reaction. The first reaction is a typical Piotrowski reaction used in the biuret method. The reaction between peptide bonds and copper ions produces coloured complexes.
The second reaction uses the Folin–Cocalteu reagent. This reagent is typically used to detect aromatic amino acids in proteins (tyrosine and tryptophan). The reaction is based on the reduction of phosphotungstic and phosphomolybdic acid salts by aromatic amino acid residues and copper complexes. The reaction takes place in an alkaline environment (pH 10). This results in the formation of coloured products. The intensity of the colour is directly proportional to the amount of protein.
The method is very sensitive. It allows the detection of as little as 1 μg/ml of protein. It is approximately 100 times more sensitive than the biuret method. Unfortunately, due to the participation of tyrosine and tryptophan in the reaction, its result depends on the amino acid composition of proteins.
Note: The reaction occurs at pH 10. Under these conditions, phosphomolybdic acid is unstable and quickly reduces to orthophosphoric acid. For this reason, the sample must be mixed quickly to ensure that the phosphomolybdic acid reacts with the protein.
The reaction produces a blue colour that can be measured at a wavelength of 650–750 nm.
3. Bradford method (Coomassie Brilliant Blue G-250 dye)
The Bradford method is one of the most commonly used techniques. It is fast and at the same time provides fairly high sensitivity. The technique is based on the reaction of Coomassie Brilliant Blue G-250 dye with proteins. The reaction, which takes place in an acidic environment, involves the formation of ionic and hydrophobic bonds with certain amino acids. Arginine reacts mainly with the dye, and to a lesser extent also histidine, lysine, tyrosine, tryptophan and phenylalanine. The resulting complex is blue with a maximum absorption at a wavelength of 595 nm. The measurement is extremely simple. Simply add the reagent to the sample and read the result after about 5 minutes. Similar to the Lowry method, Bradford method may give different results depending on the protein due to differences in amino acid composition. In addition, some detergents such as SDS, Triton-X or Tween may react with the reagent and produce an interfering colour.

BCA (Bicinchoninic Acid) method
The BCA method is a modification of the Lowry method. Like the Lowry method, it is a two-step process. In the first stage, the Piotrowski reaction takes place. Peptide bonds reduce copper ions. These react with bicinchoninic acid (BCA). The result is a stable purple-coloured complex. The absorbance of the product is measured at a wavelength of 566 nm.
The BCA method is very sensitive. It detects lower protein concentrations than the Lowry method, and at the same time its result does not depend on the amino acid composition, as is the case with the Bradford method. The disadvantage is that it is sensitive to the presence of reducing agents in the sample and to the presence of chelating agents such as EDTA.
Comparison of Protein Content Determination Methods
Individual protein determination methods differ in terms of sensitivity, speed of execution, accuracy of results, and vulnerability to interference from matrix components. The table below will help you make your choice.
| Methods | Sensitivity and range | Test duration | Accuracy | Interference |
| Absorbance measurment at 280 nm | Low (0,1 – 3 mg/ml) | Very quick | Average (depending on protein composition) | Susceptible to contamination with nucleic acids and amino acids. |
| Microbiuret method | Low (1 – 10 mg/ml) | Average (10 – 20 minut) | High (independent of protein composition) | Resistant to matrix composition |
| Lowry method | High (0,01 – 1 mg/ml) | Long (40 – 60 minut) | High (depends partly on composition) | Susceptible to contamination with detergents |
| Bradford method | High (0,005 – 2 mg/ml) | Quick (~ 5 minut) | Average (depending on protein composition) | Susceptible to contamination with detergents |
| BCA | High (0,005 – 2 mg/ml) | Long (30 – 60 minut) | High (independent of protein composition) | Susceptible to contamination with reducers |
Summary
The choice of the appropriate protein quantification method depends on the specific characteristics of the sample (e.g. the presence of detergents or interfering substances), the required concentration range, and the time and equipment available.
- UV 280 nm is ideal for quick, non-invasive preliminary measurements.
- Bradford reigns supreme for the highest sensitivity and speed (in samples without detergents).
- For the highest accuracy, I recommend the BCA method.
- The Lowry and Microbiurete methods are less commonly used, although Lowry is still used in specific applications.
Note: When selecting a measurement technique, remember to consider not only its simplicity and speed, but also its sensitivity and the composition of the samples you want to measure. Choose the method that best suits your needs.
Literature
Kłyszejko-Stefanowicz, L. (red.). (2003). Ćwiczenia z biochemii. Wydawnictwo Naukowe PWN.
Wilson, K., Walker, J. (2010). Principles and Techniques of Biochemistry and Molecular Biology.
Graphics
Helito, CC BY-SA 4.0 https://creativecommons.org/licenses/by-sa/4.0, via Wikimedia Commons
Grafika własna
