Cytotoxicity tests are among the most commonly performed tests in in vitro laboratories. They allow the detection of the impact of various factors on cell viability. Such tests are used in many fields, from toxicology to pharmacology.

Modern in vitro toxicology offers a wide range of tools for assessing the impact of chemicals, drugs and biomaterials on cell health. Choosing the right method is crucial for the reliability of results and understanding the mechanism of action.
What is cytotoxicity?
Cytotoxicity is the ability of an external agent to cause pathological changes in a cell, leading to its dysfunction or death. This process can occur through:
- Necrosis (sudden death, rupture of membranes),
- Apoptosis (programmed cell death),
- Autophagy (digestion of one’s own organelles).
These studies form the foundation for the assessment of biocompatibility in medicine and pharmacy.
Cytotoxicity versus proliferation – a crucial distinction
Although these terms often appear side by side in the literature, they describe different aspects of cell culture conditions. Understanding the difference between them is essential for the correct interpretation of research results.
- Cytotoxicity (toxic effect): Focuses on destruction. Here, we measure how many cells have died or suffered irreversible structural damage (e.g. membrane rupture in the LDH test). The result tells us about the direct harmfulness of the substance.
- Proliferation: Focuses on cell division. Here, we measure the ability of a population to increase its numbers over time.
Why is this distinction important?
The tested substance may be cytostatic but not cytotoxic. This means that a given compound (e.g. certain anti-cancer drugs) may completely inhibit cell division (no proliferation) without causing immediate cell death. The MTT (metabolic) test will show a decrease in signal, but the trypan blue test may not show dead cells.
In biomaterial research (e.g. implants), we strive for low cytotoxicity and high proliferation (we want cells to colonise the implant). In oncology, we look for substances that either kill cells (cytotoxic) or effectively inhibit their proliferation (anti-proliferative). This is extremely important when choosing a test.
Why test for cytotoxicity?
Cytotoxicity tests are performed both in basic research and in commercial laboratories. Their versatility allows them to be used in many fields and aspects.
- Studying the impact of environmental factors (e.g. food, pollutants) on cells
- Selecting drug candidates (drug discovery).
- Understanding the mechanisms of toxic effects of molecules at the molecular level.
- Replacing animal models with precise cell models (3R principle).
- Testing materials used to manufacture implants and other medical devices.
- Assessing the safety of new medical devices (ISO 10993-5).
Review of cytotoxicity tests
Researchers have access to a full range of cytotoxicity and proliferation tests. Depending on their needs, endpoint tests and tests that allow them to track culture dynamics are available. The tests also vary in terms of throughput, availability and the amount of information they provide.
The methods currently used are divided into endpoint techniques and dynamic techniques. The following summary describes the tests according to the measurement technique.
Colorimetric and fluorometric methods
- MTT/XTT/WST-1 test: These are among the most commonly used tests. Their mechanism of action is based on monitoring mitochondrial dehydrogenase activity. Mitochondrial dehydrogenase reduces tetrazolium salts to a coloured product (formazan). The amount of formazan produced is directly proportional to the number of living cells. The measurement is performed spectrophotometrically directly on multi-well plates for cell culture. The tests are proliferation tests (they show the number of cells).
- LDH test: Unlike tests based on mitochondrial dehydrogenase, the LDH test is a cytotoxicity test. During cell death, an enzyme called lactate dehydrogenase is released into the culture medium. Measuring the activity of this enzyme allows for a relative determination of the number of dead cells. The measurement is performed using a substrate that is broken down by lactate dehydrogenase. The number of dead cells is directly proportional to the colour level. The LDH test can often be performed simultaneously with a test measuring mitochondrial dehydrogenase activity to obtain a broader picture of the culture.
- Neutral red uptake (NRU) test: This test is based on the functioning of lysosomes in healthy cells. Neutral red penetrates the membranes of healthy cells and accumulates in lysosomes. The amount of neutral red taken up is directly proportional to the number of living and intact cells.
Classic observation: Trypan blue test
This is one of the oldest methods of assessing viability, based on dye exclusion. The mechanism of action is simple. Trypan blue is unable to penetrate the cell membranes of living cells. They remain unstained, while dead cells stain blue. The analysis is performed by microscopic observation, e.g. in a Burker chamber.
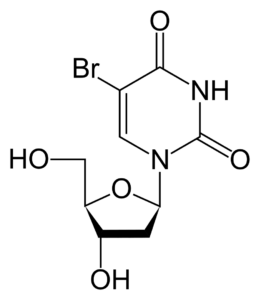
Proliferation analysis: BrDU test
The BrDU test analyses the ability of cells to divide. For this purpose, the BrDU reagent (5-bromo-2′-deoxyuridine), which is an analogue of thymidine, is used. It is incorporated into newly synthesised DNA in the S phase of the cell cycle. The BrDU level is usually determined using an ELISA or immunofluorescence test. This allows the rate of cell proliferation under the influence of the test factor to be precisely determined.
Flow cytometry (e.g. with DAPI)
Cytometry allows thousands of cells to be analysed in a matter of seconds, providing data on the entire population. Fluorescent dyes that bind to DNA, such as DAPI or propidium iodide (PI), are used. DAPI penetrates cells with damaged membranes or (with appropriate fixation) allows the DNA content in the nucleus to be assessed. It allows the phases of the cell cycle (G0/G1, S, G2/M) to be distinguished and sub-G1 (apoptotic) cells to be identified. When additional fluorescent markers are used in the flow cytometer, it is also possible to determine the level of different cells (important in mixed cultures) or to determine the level of different proteins or receptors.
Real-time monitoring: xCELLigence system
More and more companies are offering systems for real-time monitoring of cell cultures. Although quite expensive, these systems provide a wealth of information about culture dynamics and have a huge advantage over endpoint tests.
The cells are cultured on plates with gold microelectrodes. The system measures electrical impedance (resistance), which changes with cell adhesion, growth and morphology (Cell Index).
Which test should you choose? (Advantages and disadvantages)
| Method | Advantages | Disadvantages |
| Trypan blue staining | Very cheap, quick overview of the culture status. | Labour-intensive (manual), low statistical precision. Trypan toxicity may distort results. |
| MTT/XTT/WST-1 | Standard, high repeatability, high throughput, can be combined with LDH | Toxic to the cells being tested (endpoint test), provides information about living cells. |
| LDH | Standard, high repeatability, high throughput, can be combined with proliferation tests | It only measures dead cells. |
| BrDU | Direct assessment of DNA synthesis (proliferation). | Time-consuming, especially for non-adherent cells |
| Flow cytometry (DAPI) | Exceptional precision, multi-parameter analysis. | Very expensive equipment, complicated sample preparation (especially for adherent cells) |
| xCELLigence | Kinetics of changes, no dyes, non-invasive. | The high cost of disposable electrode pads. |
Application of cytotoxicity tests
- Pharmacology: Testing for organ-specific toxicity (e.g., on HepG2 lines for the liver).
- Materials engineering: Checking whether ions released from implants inhibit fibroblast growth.
- Oncology: Testing the effectiveness of chemotherapeutics in inducing apoptosis.
- Nanomedicine: Analysis of the interaction of nanoparticles with cell membranes.
Literature
- ISO 10993-5:2009 – Biological evaluation of medical devices – Part 5: Tests for in vitro cytotoxicity.
- Ke, N., et al. (2011). The xCELLigence system for real-time and label-free monitoring of cell viability. Methods in Molecular Biology.
- Strober, W. (2015). Trypan Blue Exclusion Test of Cell Viability. Current Protocols in Immunology.
- https://kmptm.pl/glp/jednostka-badawcza-dpl-cytotoksycznosc-metoda-wychwytu-czerwieni-obojetnej-nru-wg-iso-10993-5/
Graphics:
By Fvasconcellos 03:19, 13 May 2007 (UTC) – Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=2100038
