Chromatography is one of the most important and widely used analytical techniques in modern science. From medicine and pharmaceuticals to environmental protection and the food industry, its applications are extremely broad. But what exactly does it stand for? In this article you will learn how chromatography works and what it is used for.

What is chromatography?
Simply put, chromatography is a technique used to separate compounds. The name of this method comes from the Greek words chrōma (color) and gráphein (to write). It was given by the Russian scientist Mikhail Tsvet. He used a glass tube loaded with chalk to separate plant pigments and the name comes from the colored stains of pigments obtained.
The basis of any chromatographic technique is the existence of two key elements:
- Stationary (immobile) phase: This is a solid or gel that remains in a fixed location, such as inside a glass tube (column) or on a flat surface.
- Mobile phase: This is a fluid (liquid or gas) that flows through the stationary phase, carrying with it a sample of the mixture being analyzed.
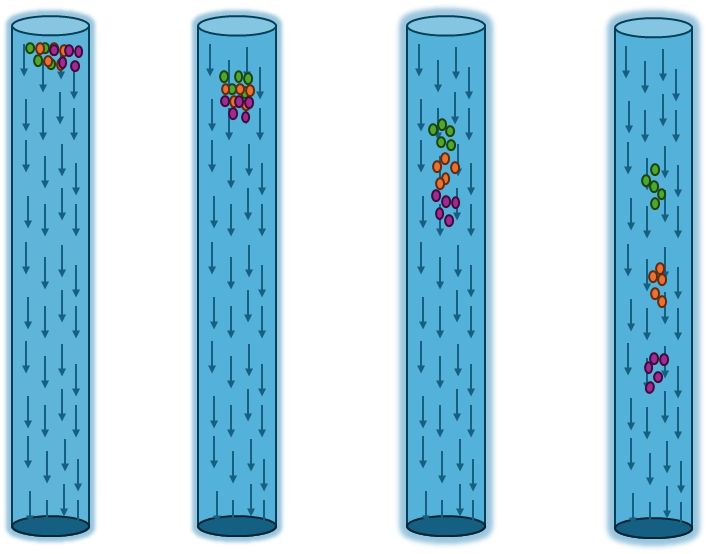
The separation of the components of a mixture is made possible by the differences in the interactions of the individual substances with the stationary and mobile phases. Components that interact more strongly with the stationary phase move slowly. Those that prefer the mobile phase move faster. As a result, after some time, the components of the mixture are separated.

Types of chromatography
Chromatography can be classified in many ways, depending on the criterion adopted. Below are the most important divisions to help understand the diversity of this technique.
Division of chromatography by the state of matter of the mobile phase:
- Gas chromatography (GC): The mobile phase is a gas (known as a carrier gas) and the stationary phase is usually a liquid applied to a solid carrier or directly to the walls of a capillary. Used for the analysis of volatile substances or those that can be easily vaporized.
- Liquid chromatography (LC): The mobile phase is a liquid (solvent or mixture of solvents). It is the most popular type of chromatography because of its versatility. A special type of it is high-performance liquid chromatography (HPLC), which uses high pressure for faster and more efficient separation.
Division of chromatography by separation mechanism:
This division is based on the mechanism of interaction between the substances being separated and the stationary phase.
- Adsorption chromatography: the separation is based on differences in adsorption (surface binding) of sample components on the surface of the stationary phase.
- Partition chromatography: Components are separated based on differences in their solubility between two immiscible liquids – a mobile phase and a stationary phase (liquid applied to a carrier).
- Ion exchange chromatography: uses a stationary phase with ionic groups that exchange ions with the analytes present in the sample. It is ideal for separating charged molecules such as amino acids, proteins or inorganic ions.
- Gel chromatography (mass exclusion): Separation is based on particle size. The stationary phase has pores of a certain size. Small molecules enter the pores and move more slowly, while large molecules are excluded and eluted more quickly.
- Affinity chromatography: This is a highly specific technique in which the stationary phase contains molecules (ligands) that bind selectively to only one specific component of the mixture (e.g., antigen-antibody).

Division of chromatography by separation technique
This division takes advantage of differences in the design of the chromatographic system.
- Column chromatography: This is the classical form in which a stationary phase is placed in a vertical glass or metal tube called a column. The mobile phase flows through the column under gravity or pressure.
- Planar chromatography: separation is done on a flat surface:
- Paper chromatography (PC): The stationary phase is a special blotting paper (chromatographic paper).
- Thin-layer chromatography (TLC): A stationary phase (e.g., silica gel, alumina) is applied as a thin layer to a glass, aluminum or plastic plate. It is a fast and inexpensive technique, often used to monitor the progress of chemical reactions.

Division of chromatography by scale of separation
Chromatography can be used in a variety of applications. It can be used to analyze material but also to purify it. For this reason, chromatography can be divided into:
- Analytical chromatography: is used as a tool to determine the composition of the sample under study.
- Preparative chromatography: is used as a tool for purifying substances in small quantities, e.g. in the laboratory.
- Industrial chromatography: serves as a tool for purifying substances in industrial quantities.

Main applications
The versatility of chromatography makes it indispensable in many fields of both science and industry.
- Pharmacy and medicine: quality control of drugs, determination of drug and metabolites in body fluids (blood, urine), diagnosis of diseases, analysis of proteins and nucleic acids. In addition, chromatography is often used for purification of active substances.
- Environmental Protection: Detection and determination of pesticides, herbicides, heavy metals and other contaminants in water, soil and air.
- Food industry: Food quality control, detection of adulteration, determination of vitamins, sugars, preservatives and food additives. Similar to the pharmaceutical industry, industrial chromatography is also used here.
- Forensics: Analysis of evidence, such as drugs, traces of gunpowder, toxins or flame accelerants. Chromatography coupled with other techniques, such as mass spectrometry, is also increasingly being used.
- Scientific research: the capabilities of chromatography are so great that it is used in many scientific studies. It can be used in chemical, biological and medical research. Its coupling with other techniques, such as mass spectrometry, MALS, makes it a very powerful tool.

Key terms in chromatography
- Analyte: The substance or component of a mixture that is being analysed (separated and determined).
- Chromatogram: A graphical record of the result of a chromatographic analysis, showing the signal from the detector as a function of time or volume of the mobile phase.
- Retention time (Rt): The time that elapses from the injection of the sample until the peak maximum of the component appears on the chromatogram. It is a characteristic of a given compound under certain conditions.
- Detector: Device located downstream of the chromatographic column that measures the concentration of analytes in the eluate and converts it into an electrical signal.
- Elution: the process of eluting sample components from the chromatographic column through the flowing mobile phase.
- Eluent: the mobile phase used in the elution process.
- Peak: Characteristic, usually Gaussian curve-shaped, maximum on the chromatogram, corresponding to a single, separated component.
Summary
Chromatography is a powerful analytical tool that has revolutionised modern science. Its ability to accurately separate even very complex mixtures means that it will remain a key technique in laboratories around the world. With its array of chromatographic techniques, it is an essential tool for scientists around the world.
Sources
Articles and books
- Skoog, D. A., Holler, F. J., & Crouch, S. R. (2017). Principles of Instrumental Analysis. Cengage Learning.
- Meyer, V. R. (2010). Practical High-Performance Liquid Chromatography. John Wiley & Sons.
- Cazes, J. (Ed.). (2009). Encyclopedia of Chromatography. CRC press.
- Wilson, I. D., Adlard, E. R., Cooke, M., & Poole, C. F. (Eds.). (2000). Encyclopedia of separation science. Academic press.
- Ettre, L. S. (2008). Chapters in the Evolution of Chromatography. Imperial College Press.
Web pages
- https://www.khanacademy.org/science/class-11-chemistry-india/xfbb6cb8de202a969:in-in-organic-chemistry-some-basic-principles-and-techniques/xfbb6cb8de202a969:in-in-purification-of-organic-compounds/a/principles-of-chromatography
- https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Instrumental_Analysis/Chromatography
- https://goldbook.iupac.org/
- https://www.waters.com/waters/en_US/HPLC—High-Performance-Liquid-Chromatography-Primer/nav.htm?locale=en_US&cid=10048919
- https://www.agilent.com/en/product/gas-chromatography
- https://www.sigmaaldrich.com/US/en/technical-documents/technical-articles/analytical-chemistry/chromatography
Graphics:
Photo by Julia Koblitz on Unsplash
Photo by National Cancer Institute on Unsplash
By Flo~commonswiki – Own work, CC BY-SA 2.5, https://commons.wikimedia.org/w/index.php?curid=436407
